Non-Invasive Prenatal Testing
Non-invasive prenatal testing (NIPT) is a blood-based genetic, prenatal screening test of the pregnant mom that screens for common chromosomal conditions that affect a baby’s health.
This non-invasive prenatal testing is indicated to perform a prenatal screening for possible abnormalities in the fetus from week 9 of pregnancy.

Find out more about the NIPT
The Non-invasive Prenatal Test consists of an analysis of the mother's blood to detect abnormalities of the fetus, avoiding performing an amniocentesis and the possible risks that this test entails.
1. Who can take the Non-Invasive Prenatal Test?
This test is indicated for pregnant women of any age from week 9 of pregnancy.
- In the following situations:
· Single or twin pregnancy
· In Vitro Fertilization Pregnancies
· Pregnancy by egg donation
· Vanishing Twin (Missing Twin)
2. What does it detect?
- Down Syndrome (Trisomy 21)
- Edwards' syndrome (Trisomy 18)
- Patau Syndrome (Trisomy 13)
- Turner syndrome (X0)
- Sex chromosomal trisomies:
- Klinefelter syndrome (XXY)
- Jacobs Syndrome (XYY)
- Triple X syndrome (XXX)
- 22q deletion syndrome (optional)
- Four additional microdeletions (optional):
- Angelman syndrome
- Prader-Willi syndrome
- Cri-du-chat syndrome
- 1p36 deletion
- Triploidy (69 chromosomes)
- Fetal sex
3. Delivery and interpretation of results
Delivery time 8 working days. * Sometimes the delivery time may be extended due to technical needs.
Interpretation:
LOW PROBABILITY: It confirms by 99% the probability that the fetus does not present the syndromes analyzed. However, it does not rule out the possibility of fetal chromosomal involvement,
HIGH PROBABILITY: To confirm a HIGH RISK result, a definitive diagnostic test is required on the amniotic fluid. It is important that you consult the results with your gynecologist.
In the following table you will find a brief summary to know the type of Non-Invasive Prenatal Test.
Laboratorio Echevarne always recommend consulting it in advance with your gynecologist.
| TPNI | PLUS | EXTENDED | SPECIAL | SPECIAL PLUS | MONOGENIC | |
| S.Down (T21) S.Edwards (T18) S.Patau (T13) |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Fetal Sex | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Triploidy | ✓ | ✓ | ✓ | |||
| Sex aneuploidies (X0, XXX, XXY, XYY) | ✓ | ✓ | ✓ | ✓ | ||
| Microdeletion 22q11.2 (Sd. Di George) | ✓ | ✓ | ✓ | ✓ | ||
| Microdeletions (Prader-Willi, Angelman, Cri-Du.Chat, Monosomy 1p36) | ✓ | ✓ | ✓ | |||
| T9, T16, T22, Aneuploidies rest of chromosomes | ✓ | ✓ | ||||
| CNV's >5Mb | ✓ | ✓ | ||||
| 92 Microdel/dup <3Mb | ✓ | ✓ | ||||
| 202 Monogenic Diseases (6246 mutations) | ✓ | |||||
| Limitations by type of pregnancy | *Not suitable: - Ovodonation - Dizygotic twins |
*Not suitable: - Ovodonation - Twins |
*Not suitable: - Ovodonation - Twins |
Non-Invasive Prenatal Test
1.What does it detect?
- Trisomy 21. S. Down:
- Trisomy 18. S. Edwards
- Trisomy 13. S. Patau
- Fetal sex
- Triploidy
2. Type of pregnancy
This test is indicated for pregnant women of any age from week 9 of pregnancy.
In the following situations:
- Single pregnancy
- Single pregnancy due to egg donation: * Triploidy is NOT reported.
- Twin pregnancy: The sex of each fetus, zygosity and fetal fraction are reported.
- Dizygotic twin pregnancy: * Triploidy is NOT reported.
- Monozygotic twin pregnancy: * Triploidy is NOT reported.
3. Interpretation of the results:
Interpretation:
LOW PROBABILITY: It confirms by 99% the probability that the fetus does not present the syndromes analyzed. However, it does not rule out the possibility of fetal chromosomal involvement,
HIGH PROBABILITY: To confirm a HIGH RISK result, a definitive diagnostic test is required on the amniotic fluid. It is important that you consult the results with your gynecologist.
4. Indications.
It is not necessary to come on an empty stomach.
Extractions from Monday to Friday.
- Price 390€
Non-Invasive Prenatal Test PLUS
1.What does it detect?
- Trisomy 21. S. Down:
- Trisomy 18. S. Edwards
- Trisomy 13. S. Patau
- Monosomy X. S. Turner
- Sexual aneuploidies
- Fetal sex
- Triploidy
- 22q11.2 S. Di George
2. Type of pregnancy
This test is indicated for pregnant women of any age from week 9 of pregnancy.
In the following situations:
- Single pregnancy
- Single pregnancy due to egg donation: * Monosomy X, Microdeletion 22q11.2, Triploidy are NOT reported.
- Twin pregnancy: The sex of each fetus, zygosity and fetal fraction are reported.
- Dizygotic twin pregnancy: * Monosomy X, 22q11.2 Microdeletion, Triploidy are NOT reported.
- Monozygotic twin pregnancy: * Triploidy is NOT reported.
3. Interpretation of the results:
Interpretation:
LOW PROBABILITY: It confirms by 99% the probability that the fetus does not present the syndromes analyzed. However, it does not rule out the possibility of fetal chromosomal involvement,
HIGH PROBABILITY: To confirm a HIGH RISK result, a definitive diagnostic test is required on the amniotic fluid. It is important that you consult the results with your gynecologist.
4. Indications.
It is not necessary to come on an empty stomach.
Extractions from Monday to Friday.
- Price 530€
Extended Non-Invasive Prenatal Test
1. Who is it for?
This test is indicated for:
- Pregnancy from week 9.
- For single pregnancy.
* Not indicated for twin pregnancy or pregnancy by egg donation.
2. What it detects?
- Trisomy 21. Down S.
- Trisomy 18. Edwards S.
- Trisomy 13. Patau S.
- Monosomy X. Turner S.
- Sexual Aneuploidies (XXX, XXY, XYY)
- Fetal sex
- 22q11.2 Microdeletion
- Monosomy 1p36
- Cri-du-Chat S.
- Prader-Willi S.
- Angelman
- Triploidy
3. Interpretation of the results:
Interpretation:
LOW PROBABILITY: It confirms by 99% the probability that the fetus does not present the syndromes analyzed. However, it does not rule out the possibility of fetal chromosomal involvement,
HIGH PROBABILITY: To confirm a HIGH RISK result, a definitive diagnostic test is required on the amniotic fluid. It is important that you consult the results with your gynecologist.
4. Indications.
It is not necessary to come on an empty stomach.
Extractions from Monday to Friday.
- Price 730€
Special Non-Invasive Prenatal Test
1. Who is it for?
This test is indicated for:
- Pregnancy from week 10.
- Twin pregnancy from egg donation.
- Pregnancy with evidence of missing twin (from week 16).
- Pregnancy if you have recently received a bone marrow transfusion.
2. What it detects?
- Trisomy 21. Down S.
- Trisomy 18. Edwards S.
- Trisomy 13. Patau S.
- Presence/absence of the Y chromosome is reported
3. Interpretation of the results:
Interpretation:
LOW PROBABILITY: It confirms by 99% the probability that the fetus does not present the syndromes analyzed. However, it does not rule out the possibility of fetal chromosomal involvement,
HIGH PROBABILITY: To confirm a HIGH RISK result, a definitive diagnostic test is required on the amniotic fluid. It is important that you consult the results with your gynecologist.
4. Indications.
It is not necessary to come on an empty stomach.
Extractions from Monday to Friday.
- Price 580€
Special Non-Invasive Prenatal Test Plus
1. What does it detect?
- Trisomy 21 (Down syndrome)
- Trisomy 18 (Edwards syndrome)
- Trisomy 13 (Patau syndrome)
- Other autosomal trisomies
- Sex chromosome aneuploidies (only in singleton pregnancies)
- Presence/absence of the Y chromosome
- 92 microdeletions/duplications >3Mb
- CNV
2. Type of pregnancy
This test is indicated for pregnant women of any age starting from week 10 of pregnancy.
In the following situations:
- Singleton pregnancy.
- Twin pregnancy: Sex chromosome aneuploidies are not reported.
- Pregnancy via egg donation.
- Pregnancy with evidence of a vanished twin before week 8 (the test is performed starting from week 16).
Check the tab "What is my NIPT?"
3. Interpretation of results:
LOW PROBABILITY: Confirms with 99% probability that the fetus does not present the syndromes analyzed. However, it does not rule out the possibility of fetal chromosomal abnormalities.
HIGH PROBABILITY: To confirm a HIGH RISK result, a definitive diagnostic test in the amniotic fluid is necessary. It is important that you consult your gynecologist about the results.
4. Instructions for the test.
Fasting is not required.
Samples are collected Monday to Friday.
- Price 810€
Monogenic NIPT
1. What does it detect?
202 dominant monogenic diseases associated with specific alterations in 155 genes.
Includes skeletal, neurological and muscular disorders, craniosynostosis and multisystem syndromes.
Includes TPNI SPECIALS PLUS.
See list of monogenic diseases
2. Type of pregnancy
This test is indicated for women with singleton pregnant women of any age from the 10th week of pregnancy until the 24th week.
Couples with advanced paternal age.
The test cannot be performed in case of egg donor, fetal death, evanescent twin or multiple pregnancy.
*Check the tab ''What is my NIPT?"
3. Interpretation of results:
NOT DETECTED: No pathogenic or probably pathogenic variant is detected within the detection range. Confirms 99% probability that the fetus does not have the syndromes tested. However, it does not rule out the possibility of fetal chromosomal involvement.
DETECTED: This test detected a pathogenic variant in one of the genes tested. This result suggests that there is an increased risk of the pregnancy being affected by the particular associated syndrome. Confirmation of a HIGH RISK result requires definitive diagnostic testing of the amniotic fluid. It is important to discuss the results with your gynaecologist.
4. Indications for carrying out the analysis.
It is not necessary to come on an empty stomach.
Extractions from Monday to Friday.
- Price 1550€
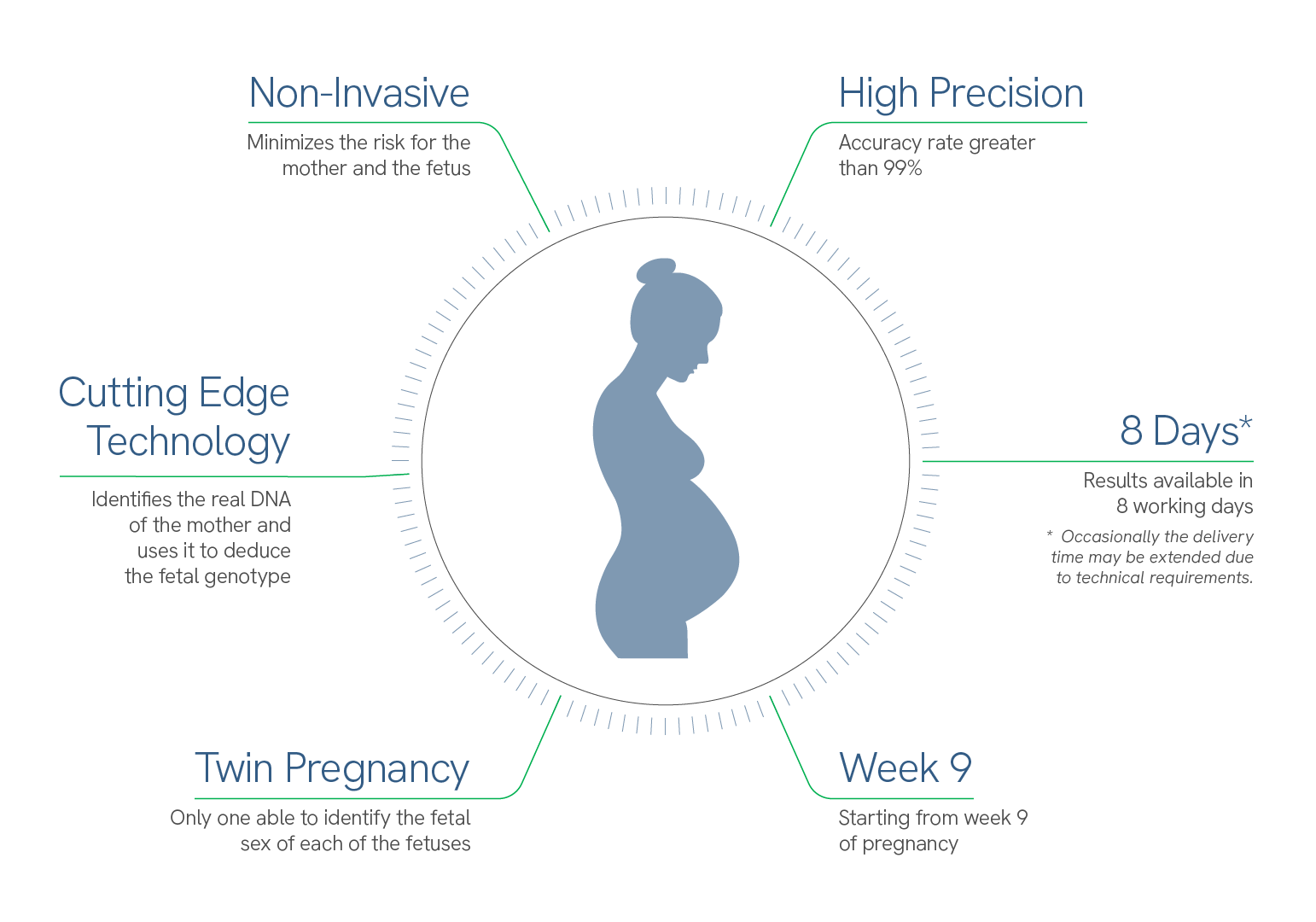
Benefits
TECHNICALLY SUPERIOR
It detects conditions that other tests cannot, including molar pregnancy, triploidy, and disappearance of the twin.
UNIQUE IN DIFFERENTIATING GENETIC PROFILE
The only test that differentiates between maternal and fetal DNA, helping to avoid false positives and false negatives.
TWIN PREGNANCY
It can assess zygosity (if identical), individual fetal sex, and individual fetal fraction
* in twin pregnancies.
NOT INVASIVE
With a simple sample of maternal blood we avoid putting the fetus at risk.
WEEK 9
The test can be done from week 9 of pregnancy.
99% PRECISION
Accuracy rate greater than 99% for the detection of Down Syndrome.